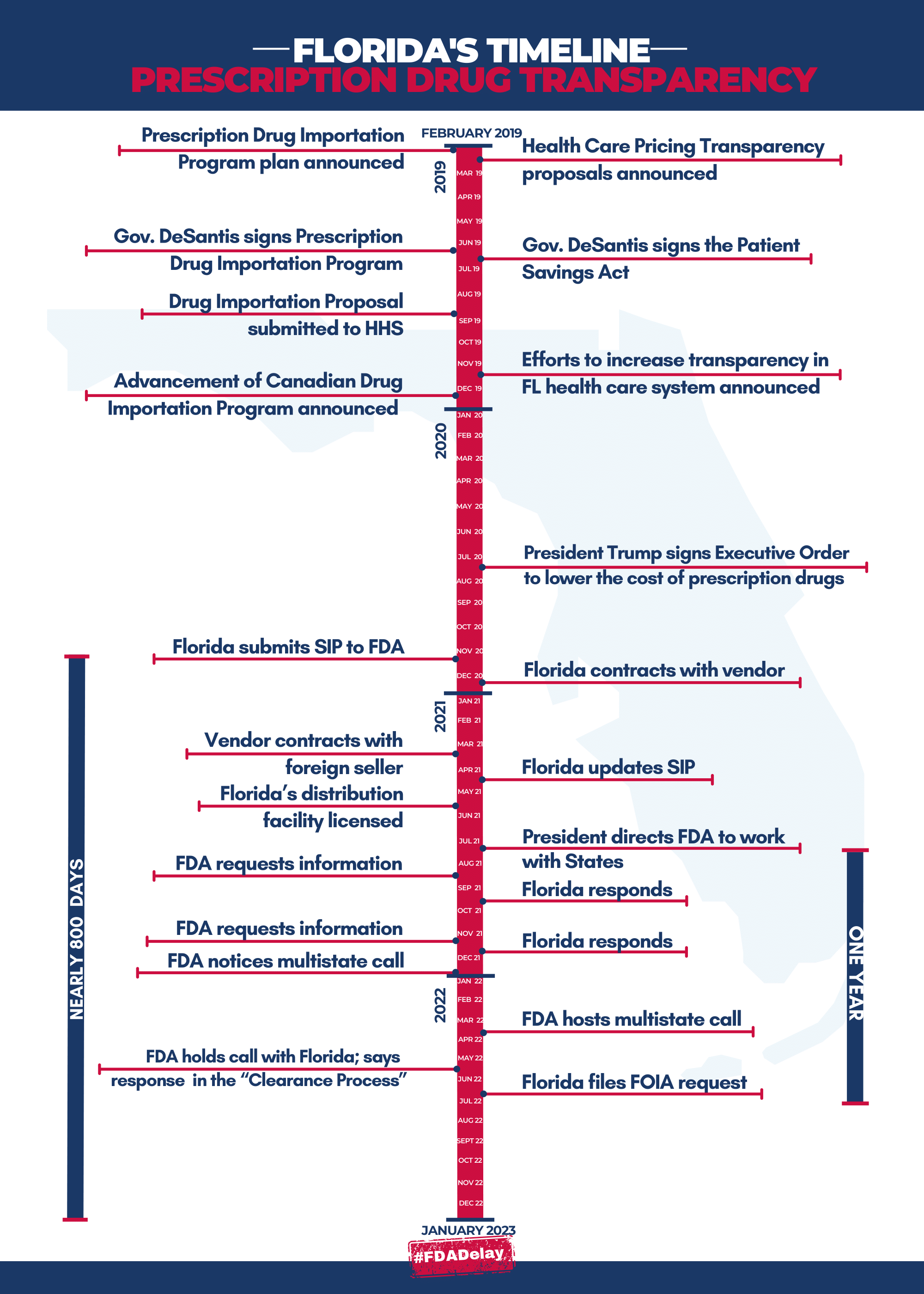
BRINGING PRESCRIPTION DRUG PRICE TRANSPARENCY TO FLORIDIANS
EXPLORE PRICE INCREASE TRENDS
Under Senate Bill 1550, drug manufacturers must report significant prescription drug price increases. AHCA publishes this information so consumers, policymakers, and researchers can better understand how drug prices are changing over time.
View DashboardMyFloridaRx
MyFloridaRx Prescription Drug Price Locator is a tool designed to make prescription drug pricing more transparent and easier to compare.
Using an interactive map, users can search by drug name and county to compare retail prices for the 400 most dispensed prescription drugs in Florida. Prices are updated monthly.
In 2026, MyFloridaRx was updated to include the Prescription Drug Price Increase Dashboard, which provides visibility into reported price increases and supports greater transparency in prescription drug pricing.
How It Works
With a simple search, you can use MyFloridaRx to find affordable prescriptions in Florida.
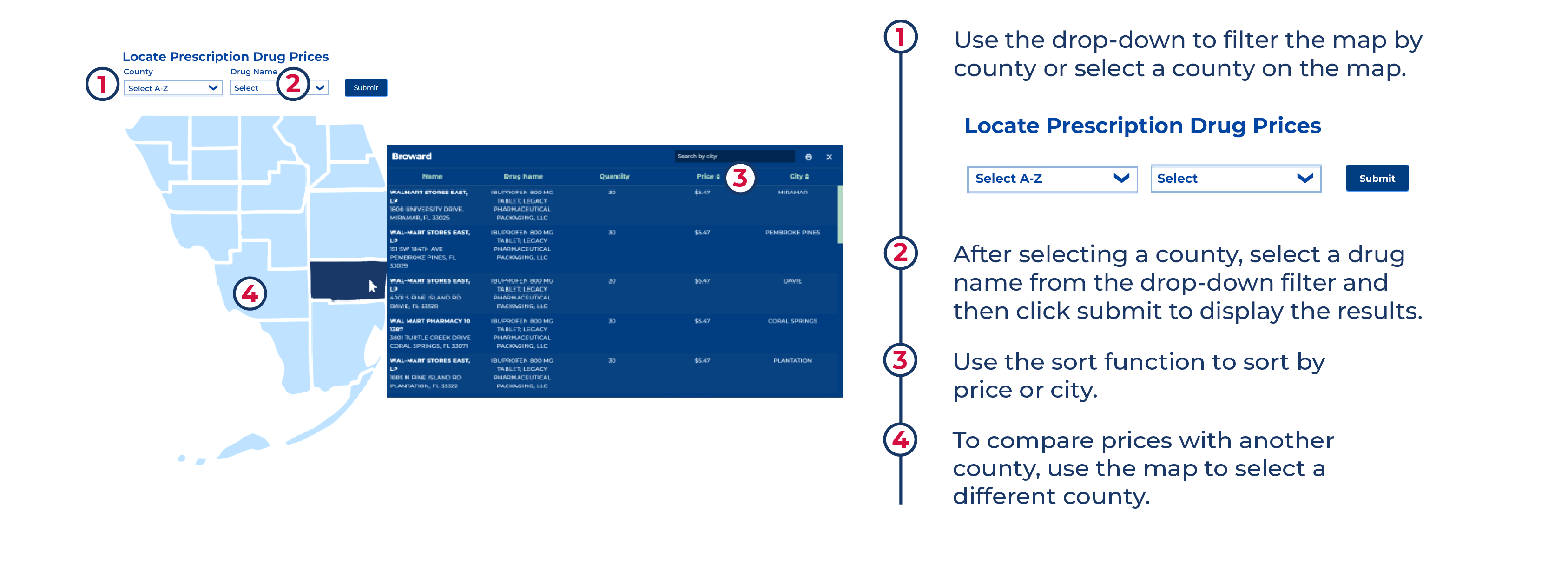
Locate Prescription Drug Prices
About This Dashboard & Methodology
These dashboard highlights prescription drug price increases reported under Senate Bill 1550 (The Prescription Drug Reform Act). Drug manufacturers must notify the Florida Department of Business and Professional Regulation (DBPR) when the Wholesale Acquisition Cost (WAC) of a prescription drug increases by:
- 15% or more within the past 12 months, or
- 30% or more within the past 3 years.
The Agency for Health Care Administration (AHCA) publishes these filings on MyFloridaRx as part of Florida’s effort to provide clear, visual insight into prescription drug pricing trends. This information helps Floridians see which drugs had qualifying list price increases and when those increases took effect.
Information on these dashboards include:
- Drugs reported since July 1, 2023,
- Manufacturer that filed report,
- Percent and dollar amount of each increase,
- WAC prior to price increase,
- Reporting threshold (15% or 30%),
- Date of price increase,
- Top ten price increases by manufacturer and drug,
- Manufacturer-level metrics and
- Drug Specific metrics.
Disclaimer: WAC is the manufacturer’s list price and does not reflect insurance, rebates, or pharmacy costs. AHCA posts this information as reported to DBPR, but does not regulate pricing. Data is published for transparency and is available for download.
Questions or comments? Contact the Florida Department of Business and Professional Regulation (DBPR)
For best viewing experience desktop is recommended.
Terminology
Wholesale Acquisition Cost (WAC)
The manufacturer’s list price for a prescription drug before any rebates, discounts, or insurance costs. WAC is not what most patients pay. It is the federally recognized price used for reporting and transparency.
Manufacturer
The company that produces and sells the prescription drug. Manufacturers are required by Florida law to report qualifying price increases. On this dashboard, “Manufacturer” reflects the licensee name submitted in the official filing.
National Drug Code (NDC)
A unique 10- or 11-digit number maintained by the federal Food and Drug Administration (FDA) for prescription drugs in the U.S. It is used for tracking, dispensing, and billing in healthcare. An NDC is made up of three segments:
- Labeler Code: identifies the manufacturer or distributor,
- Product Code: specifies strength, dosage form, and formulation, and
- Package Code: indicates package size and type.
Proprietary vs Nonproprietary Drug Name
Proprietary Drug Name: The brand name a drug is marketed under that consumers typically recognize.
Non-proprietary Drug Name: The generic name or active ingredient of the drug. It helps show what the drug is, even if marketed under a different brand. This is useful for researchers comparing drug classes.
Percentage of Price Increase
How much the WAC increased in percentage terms. For example, if a $100 drug increases to $115, that is a 15% increase. This helps compare increases across low-cost and high-cost drugs.
Dollar Amount of Price Increase
The actual dollar amount added to the WAC. This is useful for understanding impact on high-cost drugs where a small percentage change may be a large dollar amount.
Method of Increase Being Reported
Manufacturers classify the type of price increase based on Florida’s reporting thresholds: 15% increase over the previous 12 months, or 30% increase over the previous 3 years. These categories help track how often each threshold is met and identify reporting patterns.
Application Date
The date the manufacturer submitted the required price increase report to the Department of Business and Professional Regulation (DBPR). It is not the same as the effective date of the price change.
Filing/Report
A single submission by a manufacturer notifying the state of a qualifying price increase. A drug may have multiple filings over time. Each filing is individually assessed on the dashboard.
Average Price Increase
The mean percentage or dollar increase across all filings within a category (drug, manufacturer, or year). Averages are rounded for easier readability.